Blog
The Power of Process Closure in Biopharmaceutical Manufacturing
When you hear the phrase, “process closure,” what comes to mind? For many in the pharmaceutical industry, it signifies transforming open unit operational steps into closed operations, shielding materials from any external environmental influences that can threaten product quality. This focus on risk mitigation is paramount in biopharmaceutical manufacturing.
What is Process Closure and Why Does it Matter?
For over two decades, the biopharmaceutical industry wrestled with defining and standardizing process closure. The release of the 3rd edition of the Biomanufacturing Facilities Baseline Guide by ISPE in October 2023 marked a turning point. Developed by a global team of industry experts, this guide harmonizes the terminology and concepts from the BioPhorum Closed System workstream’s Closure Playbook, the ASME Bioprocessing Equipment standard, and the ISPE Baseline Guide. This guide establishes a common language between these three documents and a lexicon of common terminology, focused on a simple premise for process closure; a process is either closed or it isn’t.
The terms ‘’closed system” and “process closure” have been confusing. Before the guide, ISPE’s glossary alone included 14 different definitions of a closed system, leading to significant regulatory confusion. But keep in mind, the two key drivers of GMP compliance have never wavered; the safety of the patient and the efficacy of the product. So, why the confusion?
The Regulatory Landscape and the Q7A Guidance
The FDA’s Q7A guidance, which defines GMP for pharmaceutical manufacturing, underscores the power of process closure. “Where the equipment itself (e.g., closed or contained systems) provides adequate protection of the material, such equipment can be located outdoors.” (Section IV, 4A, paragraph 4.1). Located outdoors. Adopted and published in August 2001, this was not the starting point. This was the goal.
Historically, the term “environment” in biomanufacturing has been ambiguous, referring to everything from manufacturing suites to biosafety cabinets. Because it defines the interior space within a manufacturing vessel, the term Process Zone was introduced. “The process zone is the space that is in direct contact with the process, product, or product intermediates. So now, a closed system becomes, “A system that isolates the process zone from its manufacturing environment and prevents ingress of environmental contaminants during product contact. The process zone is limited by the equipment process boundary.’
Unleashing the Power of Closed Systems
By isolating the Process Zone from any contaminants, you now render the environment neutral, thus meeting the intent of Q7A. In doing so, your closed systems achieve more than just contamination control. They offer a range of benefits such as reduced space requirements, reduced utility consumption, lower cost-of-goods, increased operational efficiency, and enhanced flexibility.
These systems can reduce the need for large, classified spaces that require environmental monitoring, complex architecture for transitions, and often complex containment design. This translates to smaller air handling units (AHUs), reduced energy consumption, and – when air flows can be in synergy vs. opposing to reach a state of containment – easier temperature and pressure maintenance.
Reaping the Benefits for Biopharmaceutical Manufacturing
This approach leads to a focused optimization of manufacturing in multi-product operations. Whether in concurrent or campaigned operational approaches, closed systems yield many benefits:
- Simplified Segregation Strategies. Closed systems mitigate the risk of cross-contamination, simplifying workflows for personnel, materials, equipment, and waste. This reduces the need for unidirectional flows and complex airlock designs.
- Optimized Bioburden Control. Process closure involves two main steps; the removal of latent bioburden and contaminants in the previously defined Process Zone, and the mitigation of contamination ingress post-closure after the validation of closure. By creating a bacteriostatic state of all process unit operations, you can significantly reduce or optimize your cleaning and sterilization (CIP/SIP) operations.
- Reduced Complexity. By simplifying product protection elements, closed systems lead to less complex equipment designs and manufacturing processes
Case Study: Realizing Cost Savings
The power of process closure on cost savings is demonstrated in the article, “Challenging the Cleanroom Paradigm for Biopharmaceutical Manufacturing of Bulk Drug Substances.” [i] This study analyzed data from multiple organizations and found substantial reductions in both operating and capital costs due to process closure.
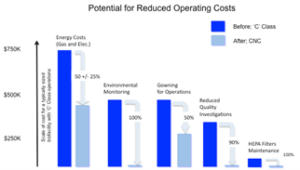
This figure shows the potential for cost savings based on a typical Bulk Drug Substance (BDS) manufacturing facility with 180 production operators, 15,000 sq. ft. of class ‘C’ space changing to ‘CNC’; air changes reducing from 30 to 10 per hour, Relative Humidity ranges changing from 45% to 60%. The data provided and analyzed is from the participating companies and covers a range of manufacturing scale and facility ages.
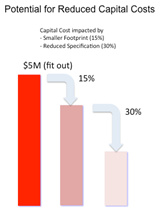
This figure shows reduced capital cost from a typical scale manufacturing facility design (15,000 sq. ft.), projected based on ‘CNC’ requirements compared to ‘C’ class requirements and a change in cost per sq. ft. of $100.
Process closure is a powerful tool that allows organizations to mitigate risk, optimize operational efficiency, and provide reductions in the cost of goods. It is a decades-old idea made for today’s biomanufacturing industry.